Chocolate is a sweet that consists of cocoa butter, sugar and other ingredients. These ingredients determine not only the taste, but also the optical properties of chocolate. This means, how light is reflected or absorbed by the chocolate. Researchers in Switzerland have recently investigated the interaction between light and different types of chocolate. And how exactly does this work?
Polarisation of light
Light can be considered an electromagnetic wave that has a propagation and oscillation direction. Unpolarized light, such as sunlight or the light from a conventional lamp, propagates and oscillates in space in all directions. However, it is possible to create a specific vibration direction of the light by, for example, placing a plate with a slit in front of the unpolarized light. In this way, any vibration direction that does not exactly match the orientation of the slit will not pass. The light coming from the other side of the plate is thus, linearly polarized. This is shown in the first image on the left. The black arrows indicate the direction of oscillation.
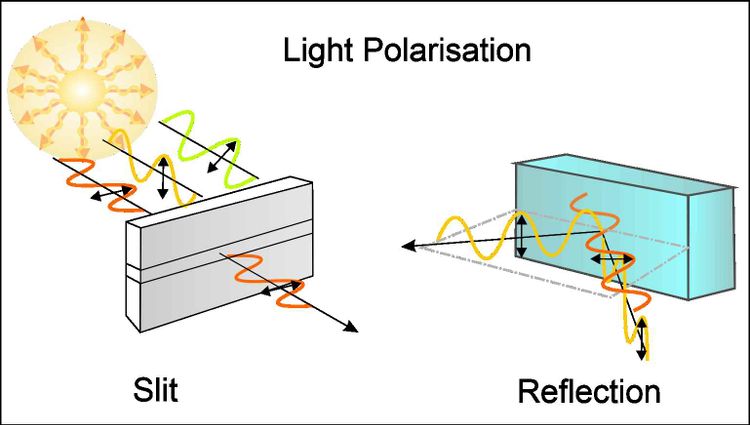
Light can also be polarised by reflection. This works particularly well at a very special angle of incidence called the Brewster angle. Under these conditions, only the light oscillating perpendicularly (vertical) to the incidence plane (marked in dot-dash lines) is reflected, while the light oscillating parallel (horizontally) penetrates the material, as shown in the picture on the right. This is how sunglasses are polarised. For this purpose, the surface of sunglasses is coated with a thin film consisting of micrometer-sized pyramids cut exactly at the Brewster angle.
When light impinges on chocolate
When polarised light falls on a surface, the reflected light will have a different polarization that the incident light, which is determined by the structure (roughness) of the surface and the material. This change in polarisation can be used to determine two important optical properties of any material: the refractive index and the extinction coefficient. The extinction coefficient is directly related to the light absorption in the material.
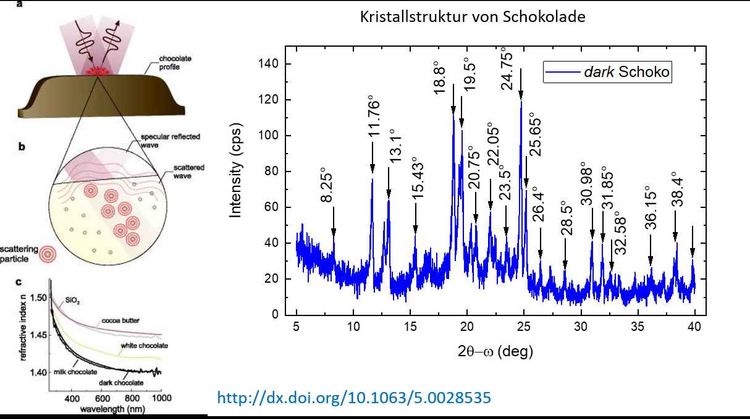
The Swiss researchers shone linearly polarized light onto different types of chocolate (white, milk and dark) and cocoa butter. From the change in polarization - from linear to elliptical - of the reflected light, they obtained the refractive index as a function of the wavelength. This can be seen in the second image on the left bottom. This shows that the refractive index of cocoa butter is very similar to that of fused silica (silicon oxide), which is a transparent material. Furthermore, the less cocoa butter or the darker the chocolate, the lower the refractive index. This means that light travels faster in dark than in white chocolate.
Colorful chocolate
In order to explain the coloration of the different types of chocolate, the Swiss researchers used the basic principle of light scattering. To do this, they created a model in which the chocolate consists of spherical cocoa butter particles embedded in a fat matrix. When light reaches the surface of the chocolate, part of it is reflected and part of it is absorbed. Inside the chocolate, the light is scattered by the cocoa butter particles. In the process, the direction of the light changes depending on the wavelength: blue light is scattered stronger than red light. This is the reason why we see the sky blue: sunlight is scattered by the molecules and atoms in the earth's atmosphere.
According to this model, the cocoa butter particles in white chocolate are approximately 400 nanometers in size. The more sugar and milk powder is added, the less light is scattered by the particles and the more white light is absorbed, which contributes to the white color. If micrometer-sized cocoa powder is added, blue and green light is scattered by the larger particles, red light is absorbed and the color changes from white to red-brown.
This means that the coloration of chocolate could be adjusted on demand by changing the size of the cocoa particles. A simpler method, according to the Swiss physicists, would be to coat the chocolate with an ultra-thin titanium oxide film. The light absorption of different colors (wavelengths) could then be controlled depending on the surface structure, and thus, blue or green chocolate could be fabricated without additional ingredients.

The structure of chocolate
The cocoa butter particles as well as the sugar in the chocolate are crystalline. This makes it possible to study the crystal structure of chocolate using X-ray diffraction. In our X-ray laboratory, we measured a piece of "Milka Dark Milk chocolate". The diffraction pattern can be seen in the second picture on the right. The structures marked with arrows in the diffraction pattern can be assigned to sugar and cocoa butter. The height and the exact position indicate the crystal structure of these ingredients.
The structure of chocolate has often been studied by X-ray diffraction and even synchrotron radiation. So far, six different crystal structures have been identified that strongly influence the melting temperature of chocolate and change it in a range between 14 and 32 degrees Celsius.
No matter which type of chocolate you prefer: Next time you enjoy a piece of chocolate, remember that it is not only delicious, but also full of physics! (Andrea Navarro-Quezada, 19.5.2021)
More blogposts
- Women in physics: Monika Ritsch-Marte, from theory to applied optics
- Gallium nitride: the allrounder among semiconductors
- Why CO2 is heating up the earth